Technical Data
How do I choose the right separation technique in liquid chromatography?
Selection based on the objective of the analysis
Firstly, it is crucial to define the objective of an analysis. The objective can already be decisive for the selection of the right separation technology.
- Size distribution of a polymer → Size exclusion SEC/GPC
- Charge variant analysis of proteins/antibodies → Ion exchange chromatography
- Analysis of small ions → Ion chromatography
- Separation of chiral compounds (enantiomer separation) → Chiral chromatography
Selection based on the chemical nature of the analytes
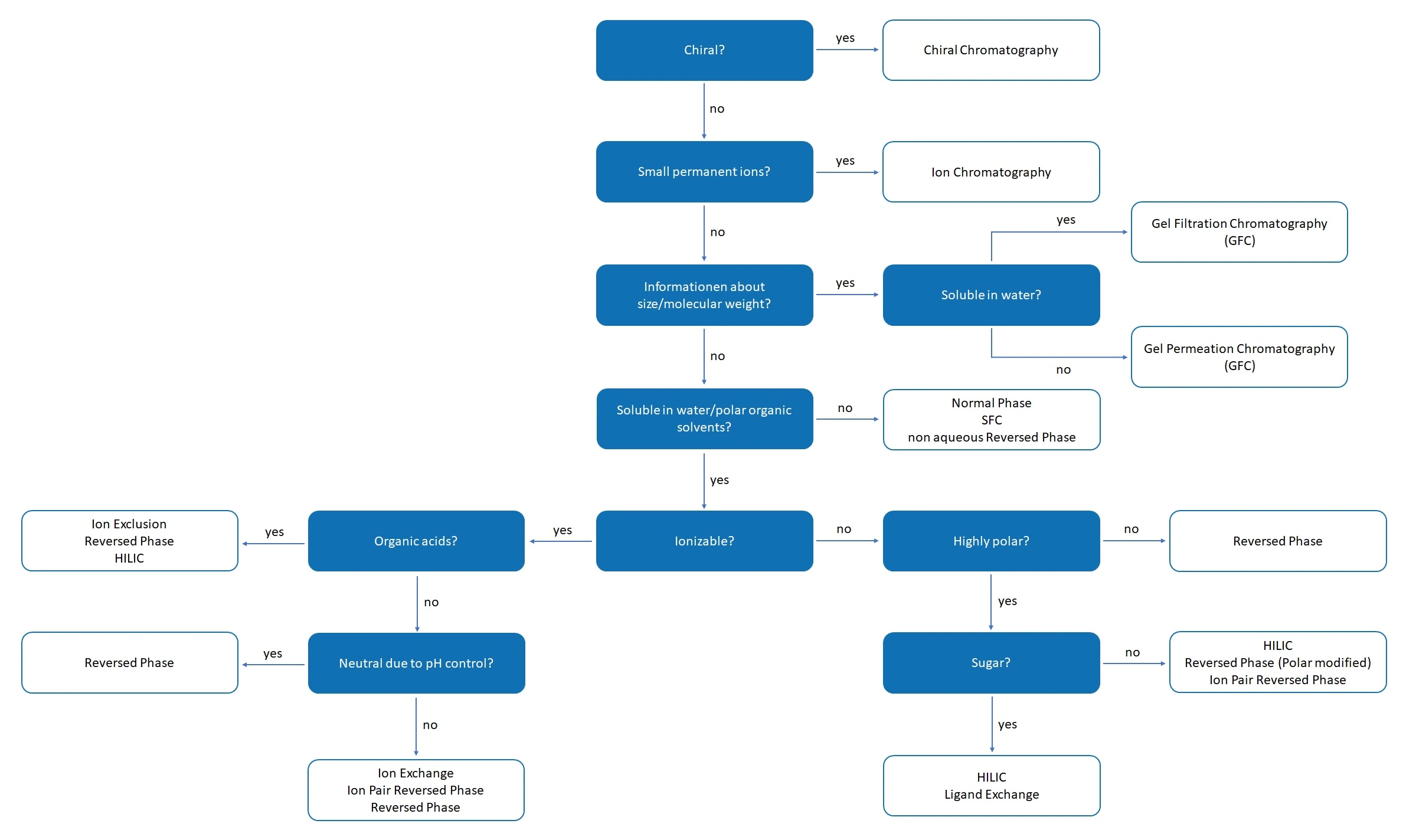
If a certain type of analyte is to be analysed, choosing the right separation technique is essential. The choice is not always clear-cut and has various advantages and disadvantages.
Organic compounds with no to very low polarity
This class of compounds includes pure hydrocarbons, lipids, carotenoids, tocopherols, etc. In principle, this includes everything that cannot be dissolved in water, methanol or other polar solvents. As solubility is only limited to non-polar solvents, separation techniques are used in which non-polar solvents are used.
- Normal phase chromatography
- Non-aqueous reversed phase chromatography
- Supercritical fluid chromatography (SFC) (disadvantage: special equipment required)
Organic compounds with low to high polarity
These include compounds such as numerous pharmaceuticals, small biological compounds, food ingredients, flavourings and fragrances, preservatives and many other substance classes. Such compounds can be dissolved in water or in mixtures of water and a polar solvent such as acetonitrile or methanol. These compounds are therefore ideal for the reversed phase. If the analytes have higher polarities, special reversed-phase columns can be used. These are polar modified columns (polar embedded or polar endcapped).
Highly polar compounds (polar or ionic)
Highly polar or ionic compounds include, for example, organic acids, amines, carbohydrates (sugars), nucleobases and nucleosides. With these types of compounds, the pH value can sometimes play a very important role (organic acids).
- Hydrohpile interaction chromatography (HILIC)
- Normal phase chromatography
- Ion pair reversed phase chromatography
- Reversed phase chromatography with polar modifications
- Special technique for organic acids: Ion exclusion chromatography
- Special technique for carbohydrates (sugars): Ligand exchange chromatography
Ionic compounds
Ionic compounds can be permanent ions or ionisable compounds. For ionisable analytes, the control of the pH value is decisive for the choice of separation technique.
- Small permanent ions → Ion chromatography
- Ionic compounds (also acids/bases) → Ion exchange chromatography
- Hydrophilic interaction chromatography (HILIC)
- Ion pair reversed phase chromatography
Compounds with amphiphilic properties
Compounds with amphiphilic properties are analytes that have both hydrophilic and lipophilic properties. This could be, for example, a charged head and a large non-polar residue. These include, for example, phospholipids and detergents. Such compounds are difficult to analyse. Mixed mode phases can be very helpful when analysing difficult analytes with different properties. The interaction can include, for example, the reversed phase and ion exchange and thus show unique selectivities.
Organic polymers
Organic polymers are biopolymers (proteins, peptides) or synthetic polymers (polyethylenes, polystyrene, etc.). Here again, the aim of the analysis plays an important role.
- Separation by size: biopolymers and water-soluble polymers → Gel filtration chromatography (GFC)
- Separation by size: water-insoluble polymers → Gel permeation chromatography (GPC)
- Separation according to charge → Ion exchange chromatography
- Separation by polar interactions → Hydrophilic interaction chromatography (HILIC)
- Separation through hydrophobic interactions → Reversed phase chromatography (RP)
- Separation of proteins by hydrophobic interactions → Hydrophobic interaction chromatography (HIC)
Chiral compounds
When separating chiral compounds (enantiomers), an achiral phase with a chiral additive can be used in the mobile phase. However, the method with a chiral stationary phase has become established and a large number of different chiral selectors are available. As the selection of a suitable phase for the separation is very difficult to predict, it is recommended that a literature search or application search is first carried out with the manufacturers. Screening services are also offered by various manufacturers. We will be happy to support you here. Please contact us!
Inorganic compounds
In HPLC, inorganic compounds usually mean ions. These are ions such as Li+, Na+, K+, Ca2+, etc. In addition to cations, anions such as Cl-, Br-, CO32-, SO42-, etc. can also be analysed.
Graphical representation of the selection guide based on the chemical nature of the analytes
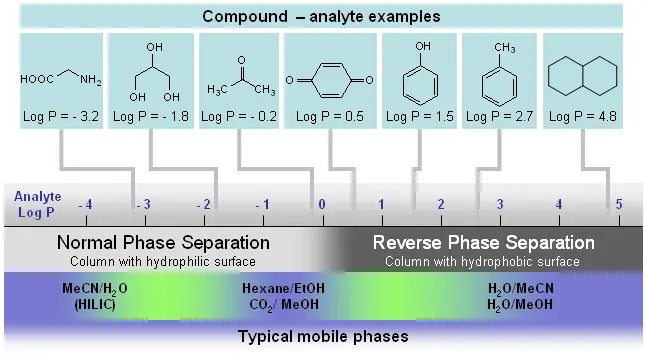
Selection based on the octanol/water partition coefficient
The octanol/water partition coefficient (P) describes the hydrophobicity of a compound. This refers to the distribution of the compound in a mixture of octanol and water.
- log P > 0: hydrophobic
- log P < 0: hydrophilic
The P values are known for many compounds or can be determined using various programmes. The log P value then determines the corresponding separation technique.
- log P < -3: Ion exchange, Ion pair reversal phase
- -4 < log P < 1: Hydrophilic interaction chromatography (HILIC), normal phase, Ion pair reversed phase
- -1 < log P < 5: Reversed phase chromatography
- log P > 5: non-aqueous reversed phase chromatography